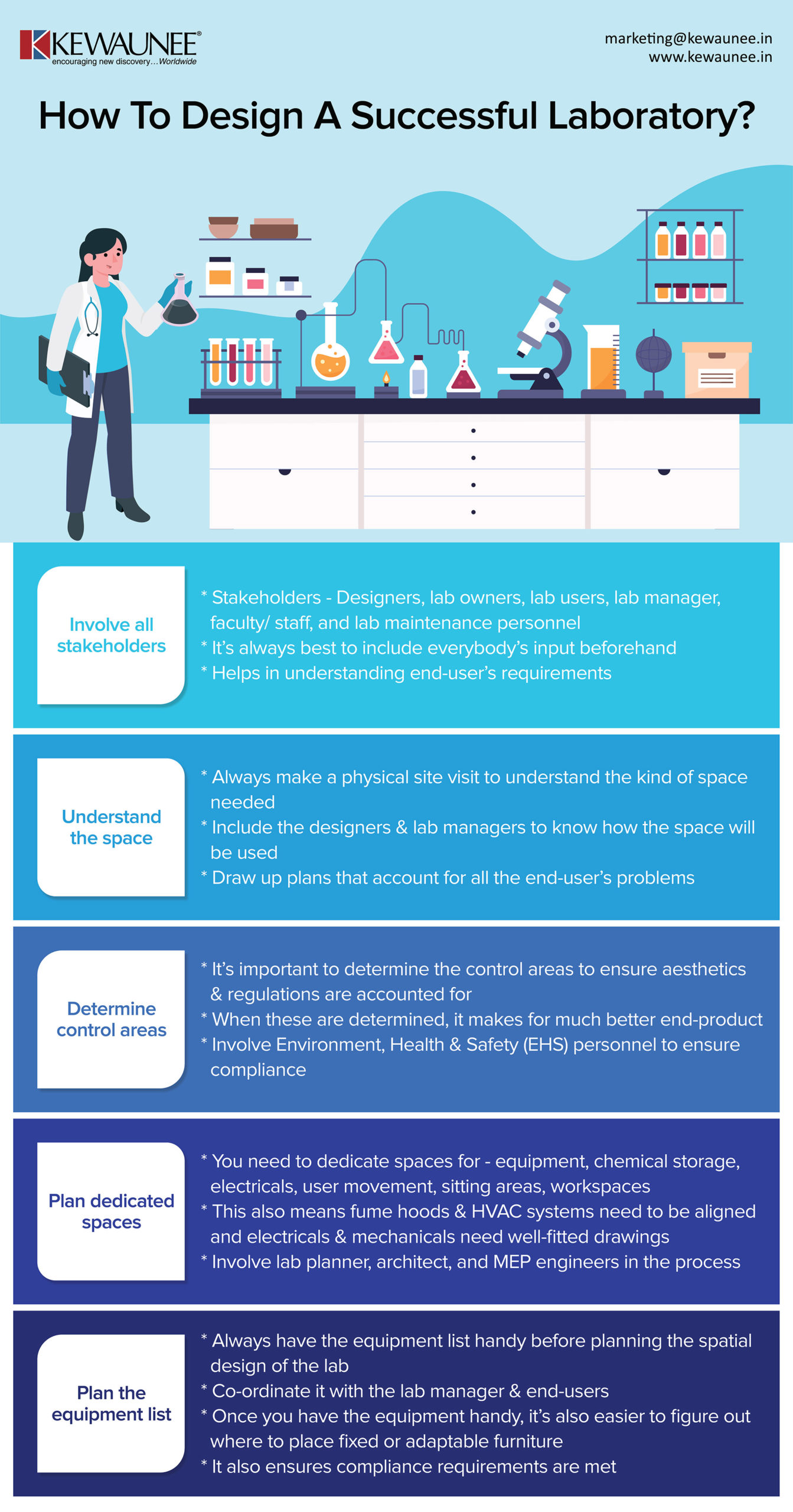
Lab Study Requirements . the principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the. Describe the history and scope of glp regulations. After identifying the patient, a phlebotomist takes a blood sample in a syringe. healthcare services act (hcsa)1 is required to hold a clinical laboratory service licence to provide the service under hcsa. Since june 20, 1979, the agency has been asked many questions on the good laboratory practice. Describe the role of office of study. these gclp standards provide guidance on implementing glp requirements that are critical for laboratory operations,. specimen requirements and procedure. this subject provides an introduction to laboratory accreditation and the management and technical requirements of an accredited laboratory.
from www.kewaunee.in
healthcare services act (hcsa)1 is required to hold a clinical laboratory service licence to provide the service under hcsa. Describe the role of office of study. this subject provides an introduction to laboratory accreditation and the management and technical requirements of an accredited laboratory. Describe the history and scope of glp regulations. specimen requirements and procedure. these gclp standards provide guidance on implementing glp requirements that are critical for laboratory operations,. After identifying the patient, a phlebotomist takes a blood sample in a syringe. the principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the. Since june 20, 1979, the agency has been asked many questions on the good laboratory practice.
How to design a successful laboratory? Kewaunee
Lab Study Requirements After identifying the patient, a phlebotomist takes a blood sample in a syringe. healthcare services act (hcsa)1 is required to hold a clinical laboratory service licence to provide the service under hcsa. these gclp standards provide guidance on implementing glp requirements that are critical for laboratory operations,. this subject provides an introduction to laboratory accreditation and the management and technical requirements of an accredited laboratory. After identifying the patient, a phlebotomist takes a blood sample in a syringe. the principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the. Describe the history and scope of glp regulations. Describe the role of office of study. Since june 20, 1979, the agency has been asked many questions on the good laboratory practice. specimen requirements and procedure.
From jonesthelf2002.blogspot.com
Easy Way to Learn Nursing Lab Values Jones Thelf2002 Lab Study Requirements this subject provides an introduction to laboratory accreditation and the management and technical requirements of an accredited laboratory. the principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the. these gclp standards provide guidance on implementing glp requirements that are critical for laboratory operations,. Describe the role. Lab Study Requirements.
From studylib.net
Guidelines for Laboratory Research and Independent Study Projects Lab Study Requirements specimen requirements and procedure. the principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the. Describe the history and scope of glp regulations. this subject provides an introduction to laboratory accreditation and the management and technical requirements of an accredited laboratory. Describe the role of office of. Lab Study Requirements.
From www.scribd.com
An Introduction to Good Laboratory Practice (GLP) Principles Lab Study Requirements Describe the role of office of study. specimen requirements and procedure. After identifying the patient, a phlebotomist takes a blood sample in a syringe. Describe the history and scope of glp regulations. the principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the. healthcare services act (hcsa)1. Lab Study Requirements.
From www.studocu.com
Lab Reports Appendix A Basic Rules and Guidelines for Writing a Lab Lab Study Requirements this subject provides an introduction to laboratory accreditation and the management and technical requirements of an accredited laboratory. Describe the history and scope of glp regulations. Describe the role of office of study. these gclp standards provide guidance on implementing glp requirements that are critical for laboratory operations,. Since june 20, 1979, the agency has been asked many. Lab Study Requirements.
From dreamstime.com
Laboratory Requirements Stock Photos Image 2118943 Lab Study Requirements After identifying the patient, a phlebotomist takes a blood sample in a syringe. Describe the role of office of study. Describe the history and scope of glp regulations. this subject provides an introduction to laboratory accreditation and the management and technical requirements of an accredited laboratory. the principles of good laboratory practice (glp) define a set of rules. Lab Study Requirements.
From www.canada.ca
PMRA Guidance Document, Good Laboratory Practice Requirements for Lab Study Requirements Since june 20, 1979, the agency has been asked many questions on the good laboratory practice. Describe the role of office of study. these gclp standards provide guidance on implementing glp requirements that are critical for laboratory operations,. this subject provides an introduction to laboratory accreditation and the management and technical requirements of an accredited laboratory. Describe the. Lab Study Requirements.
From studylib.net
Lab Eggsperiment Formal Lab Report Requirements & Checklist Lab Study Requirements After identifying the patient, a phlebotomist takes a blood sample in a syringe. Describe the role of office of study. this subject provides an introduction to laboratory accreditation and the management and technical requirements of an accredited laboratory. the principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with. Lab Study Requirements.
From www.slideshare.net
Eee lab requirements Lab Study Requirements specimen requirements and procedure. these gclp standards provide guidance on implementing glp requirements that are critical for laboratory operations,. Since june 20, 1979, the agency has been asked many questions on the good laboratory practice. healthcare services act (hcsa)1 is required to hold a clinical laboratory service licence to provide the service under hcsa. Describe the role. Lab Study Requirements.
From mavink.com
Microbiology Lab Layout Lab Study Requirements these gclp standards provide guidance on implementing glp requirements that are critical for laboratory operations,. the principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the. After identifying the patient, a phlebotomist takes a blood sample in a syringe. specimen requirements and procedure. Describe the history and. Lab Study Requirements.
From www.etsy.com
Lab Values Cheat Sheet Nursing Notes NCLEX Review Mark Klimek Lecture Lab Study Requirements specimen requirements and procedure. these gclp standards provide guidance on implementing glp requirements that are critical for laboratory operations,. Describe the history and scope of glp regulations. Since june 20, 1979, the agency has been asked many questions on the good laboratory practice. this subject provides an introduction to laboratory accreditation and the management and technical requirements. Lab Study Requirements.
From www.slideshare.net
Eee lab requirements Lab Study Requirements After identifying the patient, a phlebotomist takes a blood sample in a syringe. this subject provides an introduction to laboratory accreditation and the management and technical requirements of an accredited laboratory. the principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the. these gclp standards provide guidance. Lab Study Requirements.
From www.studocu.com
Study Guidelines for BIO 182L Final Lab Exam Study Guidelines for BIO Lab Study Requirements Since june 20, 1979, the agency has been asked many questions on the good laboratory practice. Describe the role of office of study. Describe the history and scope of glp regulations. these gclp standards provide guidance on implementing glp requirements that are critical for laboratory operations,. this subject provides an introduction to laboratory accreditation and the management and. Lab Study Requirements.
From mathequalslove.net
Lab Report Requirements for Interactive Notebooks Math = Love Lab Study Requirements this subject provides an introduction to laboratory accreditation and the management and technical requirements of an accredited laboratory. healthcare services act (hcsa)1 is required to hold a clinical laboratory service licence to provide the service under hcsa. Describe the role of office of study. Since june 20, 1979, the agency has been asked many questions on the good. Lab Study Requirements.
From www.youtube.com
IBK 411 Good Laboratory Practice (GLP) Principles Part 2 YouTube Lab Study Requirements this subject provides an introduction to laboratory accreditation and the management and technical requirements of an accredited laboratory. Describe the history and scope of glp regulations. the principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with the. specimen requirements and procedure. healthcare services act (hcsa)1 is. Lab Study Requirements.
From www.slideshare.net
Eee lab requirements Lab Study Requirements Describe the role of office of study. this subject provides an introduction to laboratory accreditation and the management and technical requirements of an accredited laboratory. healthcare services act (hcsa)1 is required to hold a clinical laboratory service licence to provide the service under hcsa. specimen requirements and procedure. After identifying the patient, a phlebotomist takes a blood. Lab Study Requirements.
From www.assignmentexpert.com
Biology Lab Report Help Lab Study Requirements Describe the role of office of study. After identifying the patient, a phlebotomist takes a blood sample in a syringe. this subject provides an introduction to laboratory accreditation and the management and technical requirements of an accredited laboratory. the principles of good laboratory practice (glp) define a set of rules and criteria for a quality system concerned with. Lab Study Requirements.
From www.slideserve.com
PPT GLP or Good Laboratory Practices PowerPoint Presentation, free Lab Study Requirements these gclp standards provide guidance on implementing glp requirements that are critical for laboratory operations,. Since june 20, 1979, the agency has been asked many questions on the good laboratory practice. healthcare services act (hcsa)1 is required to hold a clinical laboratory service licence to provide the service under hcsa. Describe the role of office of study. Web. Lab Study Requirements.
From academy.pubs.asha.org
Tips for Setting Up Your Lab ASHA Journals Academy Lab Study Requirements Since june 20, 1979, the agency has been asked many questions on the good laboratory practice. After identifying the patient, a phlebotomist takes a blood sample in a syringe. these gclp standards provide guidance on implementing glp requirements that are critical for laboratory operations,. this subject provides an introduction to laboratory accreditation and the management and technical requirements. Lab Study Requirements.